

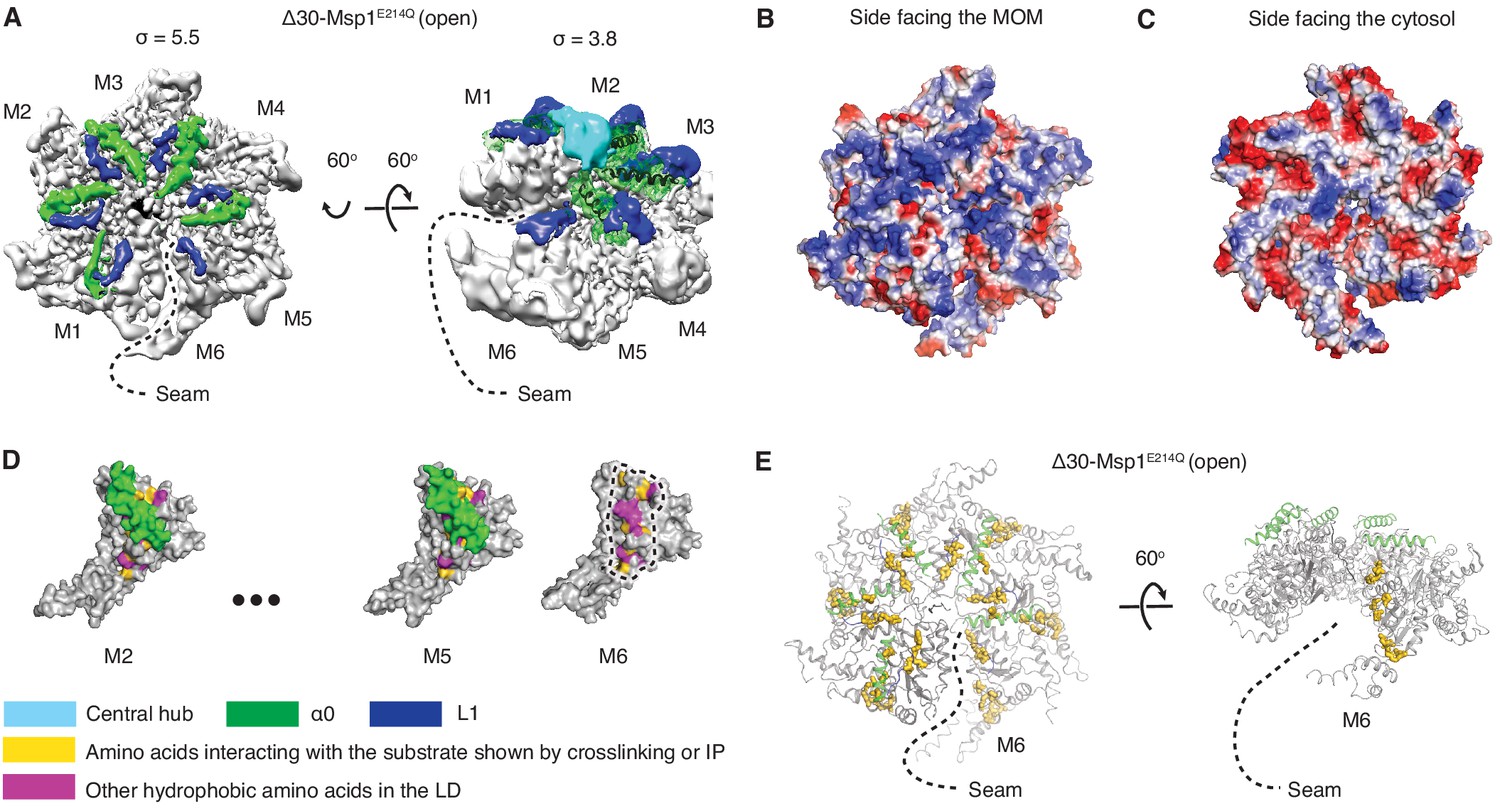
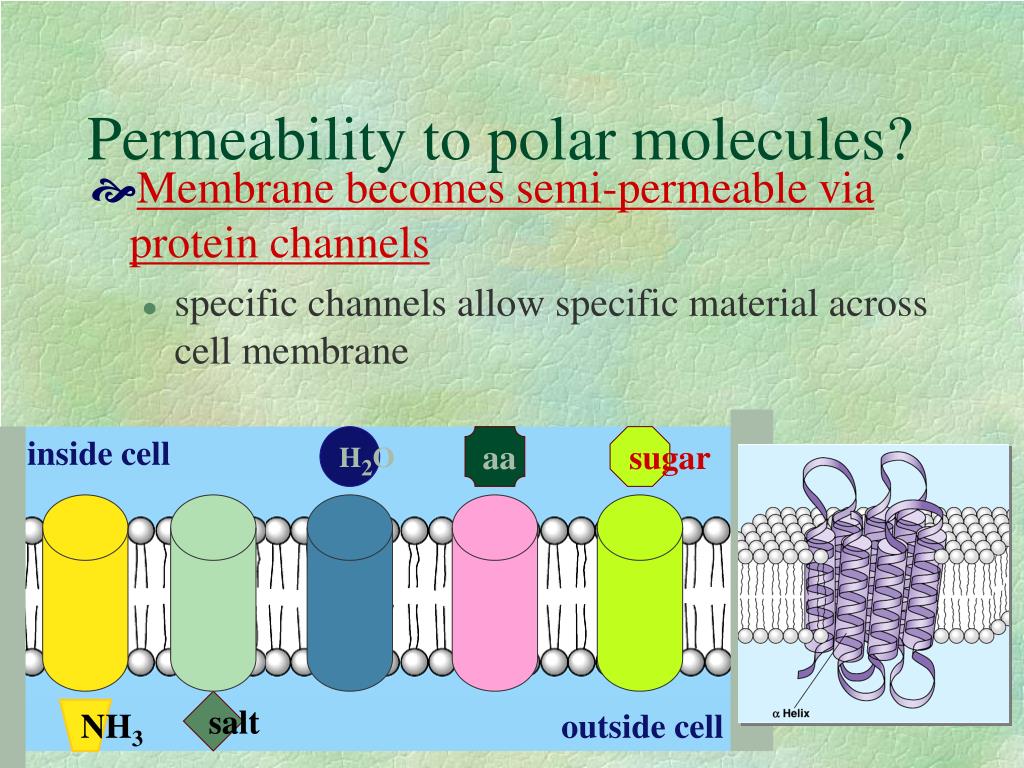
These channel pores are often formed as protein complexes that consist of several subunits. Our simulations support the idea that the hydrophobic effect contributes significantly to the stability of the closed conformation in tetrameric ion channels with a hydrophobic bundle crossing positioned in proximity to the lipid head groups of the biological membrane.Ĭellular membranes use ion channel pores to maintain cellular volume, or regulate ion flux through a process known as gating. Specifically for KcsA, these residues are part of the pH sensor important for channel gating and our results are in agreement with published electrophysiology data. Simulated mutants of amino acids within the hydrophobic region significantly contribute to the size of this difference. Its widely conserved hydrophobic bundle crossing located adjacent to the lipid head-groups at the intracellular side of the membrane was calculated to have a 5 kcal/mol free energy difference between modeled open and closed conformations. Two questions that remain unanswered regarding these channels are: (1) what force(s) stabilize the closed non-conducting channel-pore conformation? And (2) what is the free energy associated with transitioning from a closed (non-conducting) to an open (conducting) channel-pore conformation? The effects of charge and hydrophobicity on the conformational states of a model tetrameric biological ion channel are shown utilizing the amino acid sequence from the K + channel KcsA as the model “channel”. At the same time, the triggers for opening these channels, or gating, are diverse. Ion selectivity-filter structures are strikingly similar throughout the large family of K ++ channels and other p-loop-like receptors (i.e., glutamate receptors).
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA, USA.


 0 kommentar(er)
0 kommentar(er)
